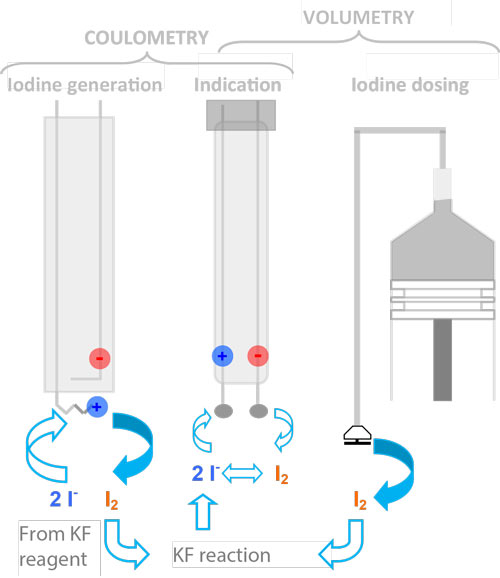
A distinction is made between the volumetric and coulometric methods. In coulometric titration, the iodine is generated directly in the titration vessel, which enables the determination of very low water concentrations. Another advantage is that the concentration of the titrant does not have to be determined.
In volumetric titration, the iodine is added precisely via a piston burette. The volumetric method is the more general method, suitable for almost all types of samples - from chemicals, petrochemicals, plastics to pharmaceuticals as well as the food industry. The samples can be either liquid, solid or gas.
The volumetric titration
In volumetric KF titration, the iodine required for the reaction is dosed into the titration cell via a burette. In the classical Karl Fischer titration, all components are offered as a combined reagent and are available in sufficient stability with suitable bases and alcohols. In addition, 2-component reagents are also offered today, in which the solvent component contains an alcohol, a base and SO2.
The titration solution then consists of an iodine solution in an alcohol. This reagent has the advantage of pH buffering and a higher concentration of all components on the left side of the reaction equation. As a result, the reaction is much faster and the reagents have a much longer shelf life. With the one-component reagent, it is possible to adapt the solvent to the solubility of the sample.
The appropriate titrator for this application is the TitroLine® 7500 KF for volumetric water determination.
The coulometric titration
Volumetric reagent addition is preferably replaced by coulometrically generated iodine at low water contents. Coulometry is based on the same chemical reaction, but the iodine is not dosed by means of a burette, but is generated in situ at the anode of a generator electrode by oxidation of iodide. Hydrogen is produced at the cathode by reduction. The amount of iodine generated is calculated by the titrator according to Coulomb's law.
Coulometry is an absolute method, therefore a titer determination is not necessary (it is also not possible!) KF volumetry and coulometry are the same except for the iodine addition.
Today, a generator electrode is used in the majority of cases without a diaphragm. Only in the case of very small amounts of water, difficult samples and when very high accuracy is needed, electrodes with a diaphragm are used. In this case, an additional catholyte is required for the cathode compartment.
The appropriate titrator for this application is the TitroLine® 7500 KF trace for coulometric water determination.
Comparison of volumetric and coulometric titration
 Today, practically all applications can be carried out easily, quickly and accurately with the coulometric and volumetric Karl Fischer titration instruments. Because of its selectivity and accuracy, Karl Fischer titration has become the most important method for water and moisture determination.
Today, practically all applications can be carried out easily, quickly and accurately with the coulometric and volumetric Karl Fischer titration instruments. Because of its selectivity and accuracy, Karl Fischer titration has become the most important method for water and moisture determination.
We would like to make the decision between a coulometric and a volumetric KF titrator a little easier for you.
In direct comparison, coulometric KF titration is simpler, since conditioning is performed automatically in the background, meaning the titration cell is kept dry.
In volumetric titration, conditioning is required for each sample before titration. Both methods complement each other and rarely can one be completely replaced by the other. Coulometry has its advantages in simple operation and the determination of very small amounts of water, while volumetry can be used far more flexibly.
In practice, there are some differences between the two methods, which are shown in the following table. The advantages of volumetry lie in the more flexible application possibilities due to different sample feeds and solvent variations. Coulometry, on the other hand, can score with lower detection limits and even easier handling.
Property | Coulometry | Volumetry |
| Water content and Sample quantity | - small water contents - small sample quantities | - medium and large water contents - adjusted sample quantities |
| Sample types | - liquid - gaseous (e.g. oven) - solid samples with oven | - solid - liquid |
| Sample addition and preparation | - with syringe directly - gas injection with oven - external extraktion - bake solid samples in the oven | - solids directly - sample mincing with homogenizer - working with increased temperature - direct with syringe |
| Operation | - very fast - very simple | - fast - simple |
| Operating range | - μg range - 10 μg to 5 mg Water | - mg range - 200 μg to 50 mg Water |
| Accuracy | - very good for small water volumes > 400 μg Water (+/- 0,5%) | - very good for water amounts > 5 mg Water (+/- 0,5% - current titer determination required |
| Reproducibility | > 400 μg Water, typical RSD approx. 1% | > 5 mg water, typical RSD approx. 1% |