The criteria are partly similar to those for a pH measurement, although the samples themselves usually have to be dissolved or diluted in water or another solvent. In a water laboratory, of course, the water itself is the sample to be analysed.
The majority of acid-base titrations take place in aqueous solutions or solutions with a high water content. If the sample does not have a very low conductivity (> 20 µS/cm) or a high viscosity, a pH electrode with platinum diaphragm can be used. The platinum diaphragm is a perfect all-rounder for many applications. Here, several platinum wires are twisted together and fused. The outflow channels between the wires have constant dimensions. Compared to the ceramic diaphragm, this ensures a pulsation-free and constant slightly higher electrolyte outflow and thus more stable measured values as well as better self-cleaning of the diaphragm.
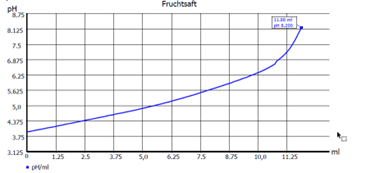
Fig. 2: Curve endpoint titration
Another important question is whether the measured pH value should be compensated by a temperature sensor during the titration. This is always important if a pH value is to be measured before the titration of the sample and the titration is to be titrated to a fixed final value(s) or if the consumption in ml is to be determined at a given pH value. Examples here are applications such as alkalinity (pH 8.3 and 4.5) or total acidity in beverages (pH 8.2). (See Fig. 2 Curve endpoint titration).
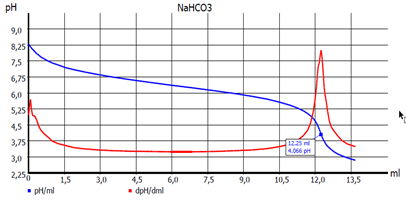
Fig. 3: EQ titration curve
In these applications, the temperature should be accurately determined during the titration. This is done either with Pt 1000/NTC 30 kOhm integrated in pH electrodes or, in individual cases, with external Pt 1000 temperature sensors.
However, if the result is determined by calculating the equivalence point(s) (EQ) from the titration curve (see Fig. 3. EQ titration), an exact determination of the temperature during the titration is not necessary. The pH value is then calculated by a manually set temperature value (e.g. 22 °C). As pH electrode with built-in temperature sensor we recommend the ScienceLine A 162-2M-DIN-ID. (see Fig. 4)
The A 162-2M-DIN-ID has the following advantages

Fig. 4: A 162-2M-DIN-ID

Fig. 5: Storage vessel Z 453
The comparable digital pH electrode for the digital measuring input of the TL 7800 TitroLine® would be the ScienceLine A 162 IDS. Of course, the A 162-2M-DIN-ID and A 162 IDS can also be used for EQ titrations.
If a pH electrode without integrated temperature sensor is to be used, we recommend the ScienceLine N 62 with plug-in head. A coaxial electrode cable such as L 1 A, L 2 A (Fig. 7) is still required for this.
External resistance thermometers W2180-Koax, W 5780 NN and W 5790 NN
A pH electrode without integrated temperature sensor can also be combined with a Pt 1000 resistance thermometer such as W 2180-Koax, W 5780 NN or W 5790 NN (Fig. 8-10). The advantage is the somewhat faster adjustment behavior of the external temperature sensor. In addition, in the event of a defect in the pH electrode, only this electrode needs to be replaced. A defect of a temperature sensor is very rare. An electrode cable L 1 NN or L 2 NN is required for the W2180 Koax.

Fig. 8: 285119030_SIA_W 2180-KOAX

Fig. 10: 285105254_SIA_W 5790 NN
Acid-base titrations in aqueous solutions with low-maintenance electrodes
Low-maintenance pH electrodes can also be used in many applications. The conductivity should not be too low (> 200 µS/cm) and the aqueous solutions should be as pure as possible.
The low-maintenance ScienceLine pH electrodes such as the A 7780, A 7780 NTC30 DIN N and A 7780 IDS are not simply low-maintenance pH electrodes with a gel electrolyte. They have three ceramic diaphragms plus a salt tablet that provides a constant supply of electrolyte to the diaphragm for two to three years. This makes them particularly robust and versatile. Even if the salt tablet is no longer visible, the electrode can still be used.
Acid-base titrations in aqueous solutions with very low conductivity or high viscosity

Fig. 13: ScienceLine N 64
For aqueous samples with very low conductivity (< 20 µS/cm), with high viscosity or emulsions, it is advisable to use a pH electrode with a ground-joint diaphragm. That would be the ScienceLine N 64 (Fig. 13). Due to the higher outflow of electrolyte, the measuring signal is set more quickly for samples with very low conductivity and the diaphragm does not clog as easily with sample solutions with a high solids content and high viscosity. The diaphragm can be easily cleaned by pushing up the ground joint.
Acid-base titrations in aqueous solutions with a complex sample matrix
A ScienceLine plus pH-electrode is recommended for aqueous samples with a demanding sample matrix, which could, for example, poison the reference system of the electrode, or where the electrolyte needs to be adjusted. All ScienceLine Plus pH electrodes have a double reference system with a silver ion barrier. This ensures universal use even in difficult samples with e.g. sulfide or protein content. The inner reference system is a maintenance-free encapsulated gel system, the outer bridge electrolyte is the proven 3 mol/l KCl on delivery. This electrolyte can be replaced by other bridge electrolytes. For the titration, either the ScienceLine Plus CPpH-A120MF plug-in head variant or variants with a fixed cable and integrated temperature sensor ScienceLine Plus pHT-A170MF-3M-DIN-N or ScienceLine Plus pHT-A170MF-3M-IDS come into question.
Acid-base titrations in aqueous solutions with small sample volumes
If only a small amount of sample is available, the titration can also be carried out with a Micro pH electrode. In addition to the
ScienceLine N 5900,
ScienceLine plus pH electrodes are also recommended here.

Fig. 16: Micro ScienceLine plus pH electrodes
The types ScienceLine Plus pH-MIC-AMF with plug-in head or the two versions with fixed cable and integrated temperature sensor ScienceLine Plus pHT-MIC-AMF-3M-DIN-N and ScienceLine Plus pHT-MIC-AMF-3M-IDS are available.
Acid-base titrations in non-aqueous solutions

Fig. 17: ScienceLine N 6480 eth and N 6480 eis
Titrations in non-aqueous solvents are all titrations in which the solvent content is > 70 %. From this moment on, no electrode with aqueous electrolytes should be used, as otherwise potassium chloride may precipitate at the diaphragm, clogging the diaphragm and thus disturbing the measurement. This would lead to very unstable measurement curves, which would make the evaluation more difficult.
Non-aqueous acid-base titrations are very often carried out as mV titrations. This means that the conversion from mV to pH is not required for these titrations. For universal use for non-aqueous titrations, we recommend the ScienceLine N 6480 eth. (Fig. 17). This is an electrode with a ground-joint diaphragm and a non-aqueous electrolyte of LiCl/ethanol.
If titrations are only carried out in glacial acetic acid (100 % acetic acid) or mixtures of glacial acetic acid and other solvents, the ScienceLine N 6480 eis (Fig. 17) can also be used. In this case, the electrolyte consists of LiCl/glacial acetic acid. However, this electrode must never be used for other non-aqueous acid/base titrations because of the acetic acid.
Acid-base titrations in non-aqueous solutions for color change
There are some acid-base titrations in non-aqueous solvents that must be performed on color change. An example would be the determination of the acid number in aviation fuels according to ASTM D3242 and the acid number according to ASTM D974.

Fig. 18: OptiLine 6
Furthermore, there are also acid-base titrations according to Eur. Pharm and USP, which are carried out on color indicator. Here we recommend the OptiLine 6 (see Fig. 18), our digital sensor for photometric titrations.
In another blog we will take a closer look at the electrodes for redox, precipitation and complexometric titrations.
Further information can be found in the titration catalog

Find here all components for a manual or automated titration with our high-precision software Titri Soft 3.5 for your use in the laboratory. Learn more about all products in the laboratory environment: coulometric and volumetric titrators, burettes, sample changers, the matching software and the corresponding titration electrodes and all accessories around titration.
Learn more
Find answers in the comprehensive titration handbook
 This is an excerpt from the titration handbook. On 192 pages it offers a compact introduction to the theory and practice of titration. If you are interested, you are welcome to download the practical guide as a PDF or request it as a brochure. And in our database you will find numerous applications for your titration for download. A concrete application example of the determination of acid in bases is described in the blogarticle "how to get correct and reproducible results in titration?". Our titration expert answers further questions from our customers in the blog article FAQ on titration.
This is an excerpt from the titration handbook. On 192 pages it offers a compact introduction to the theory and practice of titration. If you are interested, you are welcome to download the practical guide as a PDF or request it as a brochure. And in our database you will find numerous applications for your titration for download. A concrete application example of the determination of acid in bases is described in the blogarticle "how to get correct and reproducible results in titration?". Our titration expert answers further questions from our customers in the blog article FAQ on titration.
Further questions are answered by our expert in the blog article FAQ Titration. Helpful tips for your application area, you can read in our other blog articles: