 Deammonification (partial nitritation / anaerobic ammonium oxidation)
Deammonification (partial nitritation / anaerobic ammonium oxidation)
In partial nitrification, about 50% of the ammonium present is degraded to nitrite. In the second step, deammonification bacteria come into play and convert the remaining part of the ammonium nitrogen and the nitrite formed into elemental nitrogen. Since the representatives of these bacteria are anaerobic, chemolitho-autotrophic microorganisms, they require neither oxygen nor organic carbon. The oxygen requirement is reduced by 60%. Carbon is not needed at all, the saving is 100% (Figure 1).
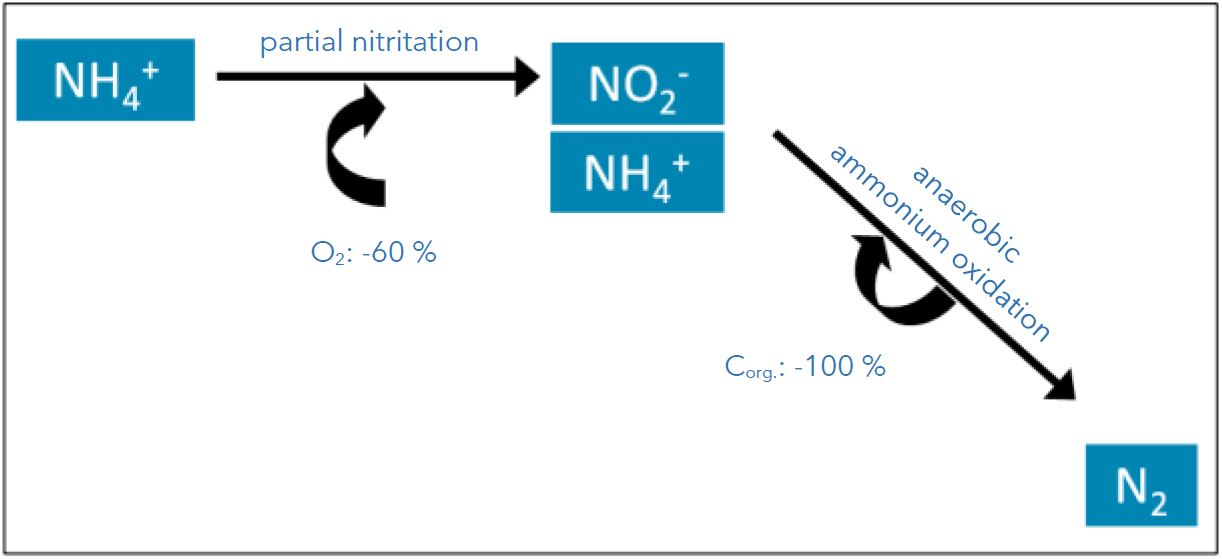
Fig. 1: Schematic representation of the deammonification, consisting of the two steps partial nitritation and anaerobic ammonium
oxidation
One- and two-stage process
Deammonification can be operated as a one-stage or two-stage process. In the single-stage process, both process steps - partial nitritation and anaerobic ammonium oxidation (anammox) - are carried out in the same basin or tank. In the two-stage process, the two steps take place in separate tanks.
In the single-stage process, an oxygen setpoint of less than 0.5 mg/l is usually selected. This is to avoid excessive nitrite accumulation and the oxidation of nitrite to nitrate. The nitrite concentration here is 2 - 25 mg/l, as too high a concentration has an inhibitory effect on the anammox bacteria. Therefore, when the upper O2 limit is reached, aeration is switched off. During the aeration phase, nitritation dominates, but anaerobic ammonium oxidation also takes place to a lesser extent.
In the two-stage process, on the other hand, oxygen setpoints of up to 1 mg/l are selected. Accordingly, higher nitrite concentrations (up to 750 mg/l) are possible.
Both methods produce nitrate nitrogen in a concentration that is about 10% of the concentration of ammonium nitrogen.
Occurrence of deammonification
During the sludge treatment of a wastewater treatment plant, nitrogen-containing process water with very high ammonium concentrations accumulates (up to 2,000 mg/l ammonium possible). Existing systems can often only inadequately cope with these considerable loads. Attempts to force sufficient nitrification often fail and result in excessively high effluent nitrate levels. Ultimately, in such cases, new approaches to wastewater treatment have to be taken to cope with the increased loads from the process water.
Challenges of deammonification
A significant difficulty in the first large-scale deammonification plants was the very time-consuming concentration of the special microorganisms, which could take up to several years. Nowadays, with the availability of well established plants with sufficiently large biomass, this difficulty has been largely eliminated. The bacteria can now also be used in larger quantities from existing plants to inoculate new plants. The running-in times of new plants are now only a few weeks. Process stability has also increased due to several years of experience, optimised measurement and control concepts and reliable measurement technology.
The biological activity of the deammonification organisms is subject to certain influencing variables, knowledge of which is imperative for a smooth deammonification process. The nitrite required for anaerobic ammonium oxidation has an inhibitory or toxic effect on the bacteria in increased concentrations, which can result in irreversible damage to the entire process. Sulphur compounds and methanol have a similar effect. With regard to a single-stage process, the oxygen required for partial nitrification simultaneously leads to the inhibition (reversible) of the organisms. Anaerobic ammonium oxidation thus requires sufficiently long phases with very low oxygen concentrations.
Nitritation and anaerobic ammonium oxidation have an opposite effect on the pH value. Aeration only occurs within a very narrow pH interval. During this phase, nitritation dominates over anaerobic ammonium oxidation. The nitrite formed causes the pH value to drop until the lower threshold value (approx. at pH 7.00) is reached. The aeration is switched off, the O2 concentration drops and the nitrite formed is used up to oxidise the ammonium still present (anaerobic ammonium oxidation). The pH value increases both through this process and through the continuous addition of alkaline process water up to the upper threshold value, which in turn leads to the start of aeration.
In addition, too low a temperature and too high a solids content lead to increased formation of NO3. This in turn results in an inhibition of anaerobic ammonium oxidation.
Usable measurement technology
Besides the most common parameters pH and oxygen, monitoring and control variables also include nitrogen concentrations (NH4-N, NO3-N, NO2-N), temperature and dry matter. Xylem offers suitable digital sensors for these parameters, all of which can be connected to the IQ SENSOR NET measuring system.
pH: SensoLyt® 700 IQ
O2: FDO® 700 IQ or TriOxmatic® 700 IQ
Nitrogens: ISE sensors, e.g. VARiON® 700 IQ
TS: ViSolid® 700 IQ
Temp: integrated in various sensors
System: IQ SENSOR NET